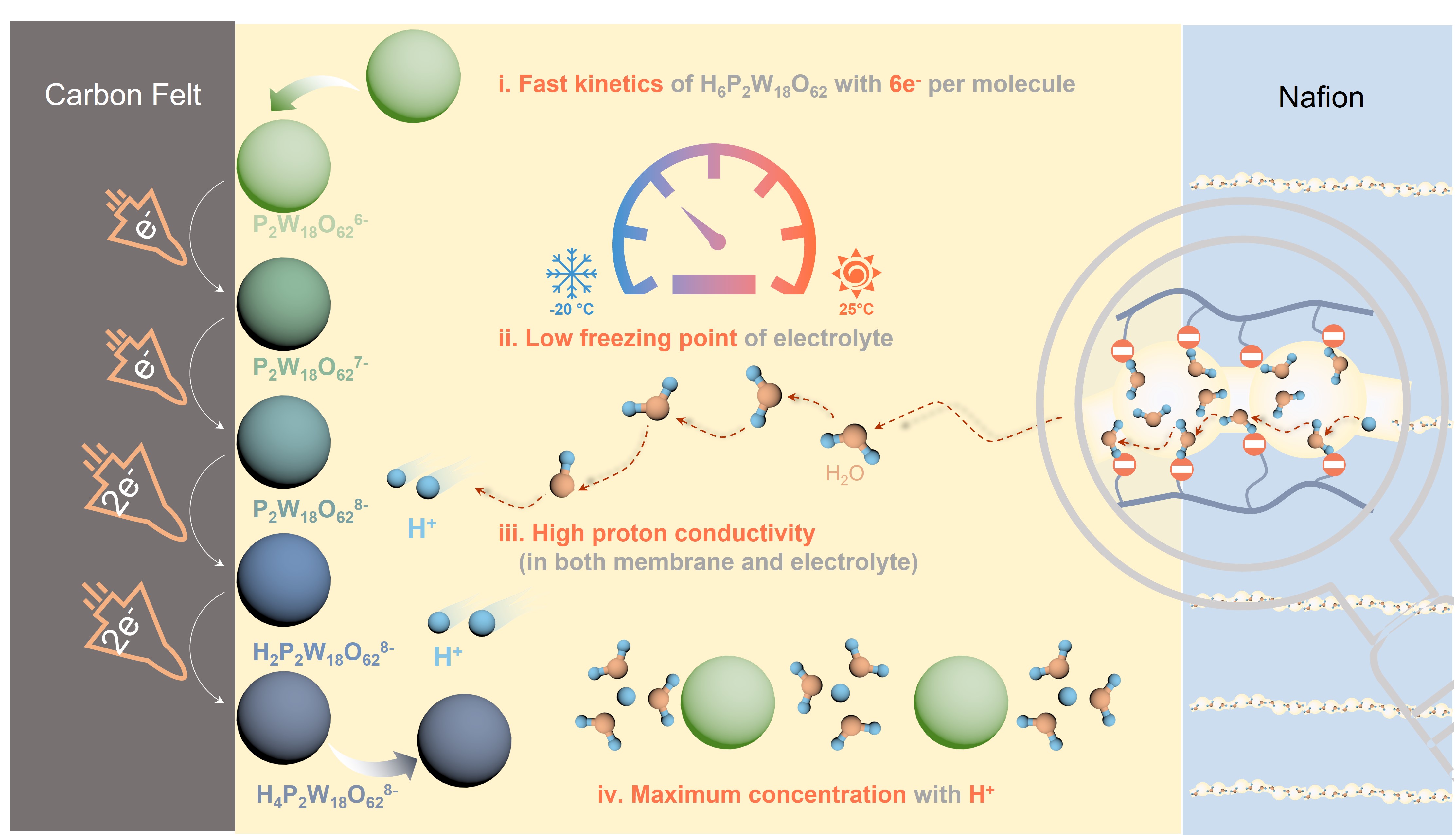
A research team led by Professor Yi-Chun Lu, Associate Professor, Department of Mechanical and Automation Engineering (MAE) at The Chinese University of Hong Kong’s (CUHK), has successfully developed a new electrolyte that enables high power, long life flow battery applications at both room temperature and low temperatures down to –20℃. The new flow battery achieves a high power density of 282.4 mW cm-2 and stability over 800 cycles (more than 1,200 hours) without decay at –20℃. This work enables high power, long life redox flow batteries to be used in regions with cold weather or severe weather fluctuations, a significant step towards the practical application of redox flow batteries for grid-scale storage of renewable energy. The breakthrough has been recently published in Nature Energy, one of the world’s leading scientific journals. Aqueous redox flow batteries are a promising technology for safe, long duration energy storage and are key to achieving massive utilisation of intermittent renewable energies such as solar and wind power. However, at temperatures below freezing, redox flow batteries cannot be used because of the freezing of aqueous electrolytes, low reaction rate and the limited solubility of active materials. These challenges not only preclude the use of redox flow batteries in cold weather regions but also make renewable electrical grids extremely vulnerable to severe weather fluctuations. For instance, the recent power failure in Texas caused by a devastating snowstorm affected many millions of people, highlighted the need for a more robust electrical grid and energy storage over a wide range of working temperatures. The state-of-the-art vanadium redox flow batteries suffer from lower solubility and lower redox kinetics at decreasing temperatures. Therefore, most commercial redox flow batteries need to use expensive, energy-consuming heating systems for low temperature applications. Professor Lu and her team describe a new active material, multi-electron heteropoly acid H6P2W18O62 (HPOM), which enables high power, long life aqueous redox flow batteries below freezing. The HPOM exhibits a low freezing point (–35℃) and a high conductivity (74.32mS cm–1 (–20℃)) , which makes it an ideal active material candidate for high-power-density flow battery application at low temperatures. The new HPOM based redox flow batteries demonstrated a high capacity, record stability (more than 1,200 hours without decay) and power density (282.4 mW cm–2) at a low temperature of –20℃, which makes them the first high-power low-temperature redox flow batteries with industrially relevant cycling stability. The full research paper can be found at: https://doi.org/10.1038/s41560-022-01011-y |
|